An X (Twitter) thread on cognitive neuroscience factors and mechanisms led to an invitation to post a reply in the new Journal of Cognitive Neuroscience forum. The forum is an interesting experiment to try to achieve some of the velocity of social media in a formal academic publishing environment, complete with DOI's. However, the forum's policy excludes non-academic participants, so it was rejected after a couple weeks of consideration. I am therefore posting the article here instead.
If you wish to compare this essay with the insight progress made by the cognitive neuroscience ingroup community, there is a BlueSky thread. Two conjectured factors blossomed into a wealth of conjectured factors and mechanisms. For me, back to building commercial products and services around the physical brain connectome as revealed brick-by-brick through experiments.
In a recent article, What do you mean when you say "one versus two factors"? And how does it map onto "computation"?, Rust and Corlett relate their joint exploration of a conceptual mapping incongruency between Rust’s expertise in familiarity and computation, and Corlett’s direct study of Capgras syndrome. On the one hand, there are psychological factors, defined as “anatomically and informationally encapsulated processing modules in the brain”. On the other, there are computations or processes, a set of crosscutting capacities that may help implement each factor, such as prediction error and metacognition.
The issue of aligning these dimensions came up while trying to articulate the neural mechanisms of Capgras syndrome and Fregosi syndrome. These are delusions in which familiar persons appear to be imposters, and strangers appear to be a familiar person, respectively. A leading theory of delusion proposed by Max Coltheart suggests that the delusion can be broken down into two factors: an impairment of “familiarity” and an impairment of “familiarity belief” evaluation. Another model holds that these two factors are instead intertwined as a single factor. But if they are intertwined, are there one or two computations involved? And where does metacognition come in?
As an outsider who has been independently compiling and annotating the neuroscience literature for the past several years, I appreciate the opportunity to weigh in with what I hope is a productive and orthogonal perspective. This reply is of more general scope than the original article, and should not be taken as directed criticism. The central theme here is that a well-manicured annotated connectome, densely interlinked with curated experimental neuroscience literature, may allow us to bypass a lot of this taxing taxonomical effort. We could generate intuitive, biologically-constrained working hypotheses by consulting with such a resource, rather than necessarily starting inquiries the usual way; i.e. from top-down mental constructs (e.g. “familiarity”, “familiarity belief”, “metacognition”), their definitions, and their operationalizations.
Effectively leveraging this requires an inversion of a widely held goal of neuroscience, which is to discover the psychological functions of brain parts. Instead of functions, where a function is a consensus abstract mental concept like ‘salience’, ‘attention’, or ‘System 1 thinking’, we might instead seek to aggregate behavioral effects of brain parts from the bottom-up, from the neuroscience literature itself, into part-by-part profiles. These profiles may accelerate insight, so that we do not have to keep writing phrases like ‘emergent complex dynamics’ as a placeholder for real understanding. The approach is best illustrated by the concrete example used in the original article.
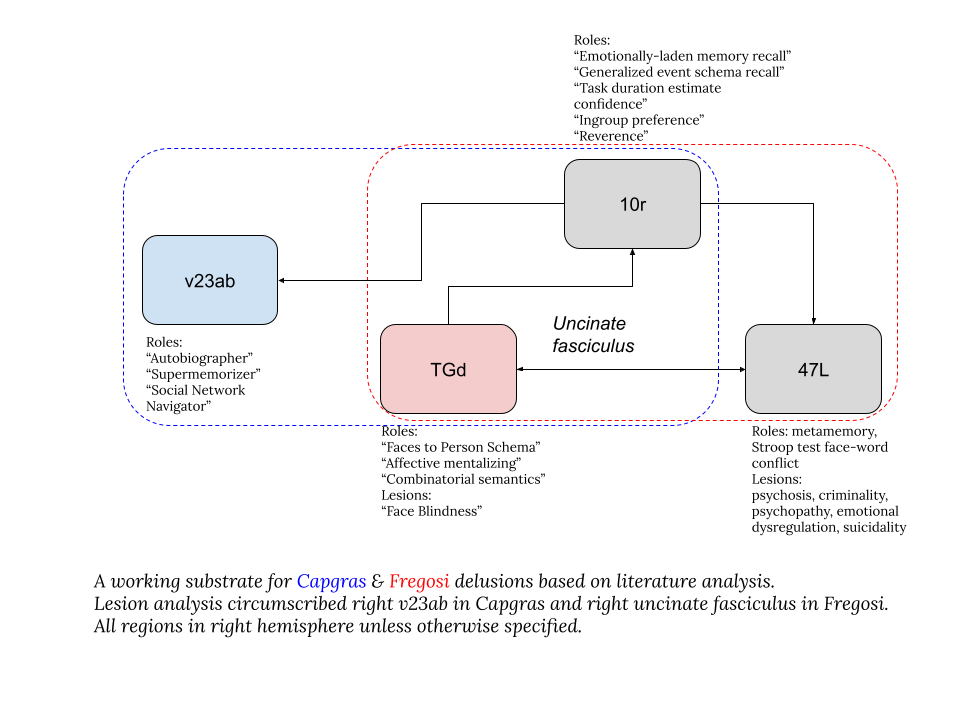
Note: in the following we are using the HCP parcellation(Glasser, et al., 2016)and a neurosurgical atlas (Baker, et al., 2018).
Let us now consider Fregosi syndrome. It is known that face agnosias tend to be right-lateralized (Verosky & Turk-Browne, 2012). Lesion analysis of Fregoli syndrome (Kakegawa, Isono, Hanada, & Nishikawa, 2020) identified damage the right uncinate fasciculus (UF) tract. Of the brain areas connected via UF, right TGd is an obvious suspect. Why right TGd? Because it seems to associate faces to people, e.g. (Borghesani, et al., 2019).
Another endpoint of UF is right 47L, corresponding roughly to the right pars orbitalis. Reduced gray matter in this area has been associated with psychotic delusion and metamemory errors (Kenney, et al., 2017), and impairment of self-belief questioning in schizophrenia (Orfei, Piras, Macci, Caltagirone, & Spalletta, 2013).
Now consider Capgras syndrome. Both lesion network analysis and familiarity meta-analysis identified analysis identified v23ab and 10r and as common connectivity nodes across the wide dispersal of brain injuries that resulted in Capgras syndrome. Area v23ab is associated with autobiographical recall (D'Argembeau, et al., 2014)and social network navigation (Zhang, et al., 2022). Area 10r is associated with generalized event schema recalls(Spalding, Jones, Duff, Tranel, & Warren, 2015), receives inputs from TGd, and projects to both v23ab and 47L. Interestingly, in one lesion network analysis, damage to 10v actually reduced delusions (Warren, Jones, Duff, & Tranel, 2014).
The above analysis is ad hoc, but weaves together existing knowledge to build an intuitive starting picture for a pair of phenomena (Capgras syndrome and Fregosi syndrome). The imagined “familiarity” factor might map to the right 10r-v23ab ensemble, and the imagined “familiarity belief” evaluation failure may map to the right 10r-47L ensemble. The imagined “metacognition” computation deficit may specifically relate to right 47L. The basic failure to map a face to a person may relate to right TGd, which is upstream of 10r and 47L. Fregosi syndrome may be more related to the frontotemporal part of the network, while Capgras syndrome may be more related to the ventral posterior cingulate. It is critical to note that these fuzzy maps are not general assignments of function, but an attempt to retrospectively relate the abstract factor-based model to a physical one, so that we can proceed with the physical one. The Taoist sage Zhuangzi advised us that words are like the rabbit trap: once one has the caught the rabbit, one ought to discard the trap.
There are many published warnings against centering anatomy in this way, mainly by theoreticians invested in mental constructs. They invoke cautions like the mereological fallacy, the anthropomorphic fallacy, biological essentialism, and neural degeneracy, and a general aversion to committing to what a brain area “does” in terms of psychological functions. These are worthwhile considerations, but the developmentally-specified distinct brain areas remain facts of nature that cannot forever remain inscrutable.
Pessoa et al (Pessoa 2022) called out the elephant in the room when they wrote, “mental terms-such as perception, cognition, action, emotion, as well as attention, memory, decision-making-are epistemically sterile.” Pessoa pointed to a way forward in his book, The Entangled Brain (Pessoa L. , 2022) : “…our understanding of the role of a specific region needs to be gradually bootstrapped so that eventually we will have a better appreciation for its functional contributions and repertoire, as we gain insight into how it interacts with other regions.” To execute on this approach requires painstaking annotation work over a long period.
It is not clear if a given brain part, and its internal and external community, should be ontologically classified as a factor, computation, function, mechanism, or module. These have not proven to be good ways to “cut nature at its joints”. If they accurately mapped the territory, we could look up keywords in Neurosynth or the Cognitive Atlas and find answers. Surely 21st century cognitive neuroscience ought to involve treating the hundreds of molecularly-specified brain parts as headliner actors worthy of characterization in themselves, rather than continuing to cast wide swathes of cerebral cortex as mere supporting actors in movies starring aging abstract mental nouns.
References
Baker, C., Burks, J., Briggs, R., Conner, A., Glenn, C., Sali, G., . . . Sughrue, M. (2018). A Connectomic Atlas of the Human Cerebrum-Chapter 1: Introduction, Methods, and Significance. Oper Neurosurg (Hagerstown). doi:10.1093/ons/opy253
Borghesani, V., Narvid , J., Battistella, G., Shwe, W., Watson, C., Binney, R., . . . Miller, B. (2019). "Looks familiar, but I do not know who she is": The role of the anterior right temporal lobe in famous face recognition. Cortex. doi:10.1016/j.cortex.2019.01.006
Darby, R., Laganiere, S., Pascual-Leone, A., Prasad, S., & Fox, M. (2017). Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain. doi:10.1093/brain/aww288
D'Argembeau, A., Cassol, H., Phillips, C., Balteau, E., Salmon, E., & Van der Linden, M. (2014). Brains creating stories of selves: the neural basis of autobiographical reasoning. Soc Cogn Affect Neurosci. doi:10.1093/scan/nst028
Glasser, M., Coalson, T., Robinson, E., Hacker, C., Harwell, J., Yacoub, E., . . . Smith, S. (2016). A multi-modal parcellation of human cerebral cortex. Nature. doi:10.1038/nature18933
Kakegawa, Y., Isono, O., Hanada, K., & Nishikawa, T. (2020). Incidence and lesions causative of delusional misidentification syndrome after stroke. Brain Behav., 10(11):e01829. doi:10.1002/brb3.1829
Kenney, J., McPhilmy, G., Scanlon, C., Najt, P., McInerney, S., Arndt, S., . . . Cannon, D. (2017). The arcuate fasciculus network and verbal deficits in psychosis. Translational Neuroscience. doi:10.1515/tnsci-2017-0018
Orfei, M., Piras, F., Macci, E., Caltagirone, C., & Spalletta, G. (2013). The neuroanatomical correlates of cognitive insight in schizophrenia. Soc Cogn Affect Neurosci. doi:10.1093/scan/nss016
Pessoa, L. (2022). The Entangled Brain. Cambridge, MA: MIT Press.
Pessoa, L., Medina, L., & Desfilis, E. (2022). Refocusing neuroscience: moving away from mental categories and towards complex behaviours. Philos Trans R Soc Lond B Biol Sci. doi:10.1098/rstb.2020.0534
Puelles, L. (2024). Functional Implications of the Prosomeric Brain Model. Biomolecules. doi:doi.org/10.3390/biom14030331
Spalding, K., Jones, S., Duff, M., Tranel, D., & Warren, D. (2015). Investigating the Neural Correlates of Schemas: Ventromedial Prefrontal Cortex Is Necessary for Normal Schematic Influence on Memory. Journal of Neuroscience. doi:10.1523/JNEUROSCI.2767-15.2015
Verosky, S., & Turk-Browne, N. (2012). Representations of facial identity in the left hemisphere require right hemisphere processing. J Cogn Neurosci. doi:10.1162/jocn_a_00196
Warren, D., Jones, S., Duff, M., & Tranel, D. (2014). False Recall Is Reduced by Damage to the Ventromedial Prefrontal Cortex: Implications for Understanding the Neural Correlates of Schematic Memory. Journal of Neuroscience. doi:10.1523/JNEUROSCI.0119-14.2014
Zhang, L., Chen, P., Schafer, M., Zheng, S., Chen, L., Wang, S., . . . Huang, R. (2022). A specific brain network for a social map in the human brain. Sci Rep. doi:doi.org/10.1038/s41598-022-05601-4



